Imaging Core of YM Campus
- Update Date:2025-05-29
- Units:Instrumentation Resource Center
- 2024/08/22 [LSM700 Upgrade] Attention: As the LSM700 parts have been discontinued, to avoid months of temporary downtime during future failures, the center will be upgrading the equipment from late September to early October (one week in advance), so please plan and arrange your experiments in advance to minimize the impact of downtime.
After the upgrade, the quality and speed of image acquisition will be improved. However, the acquisition software, scan area, intensity, etc., will differ (it cannot be applied to the original LSM700 settings), and the original users must be re-qualified to use the upgraded model. The center will arrange the training course and assist in re-qualifying. - 2024/02/01 The 2022-year application for MetaMorph has expired this year. If you need to continue to use it, please re-submit the MetaMorph IP certification application form. If you need assistance, please get in touch with Pei-jun Chen or Yung-yu Lu (ext. 65980/66185)
- 2023/01/18 The visible light source of the Leica DM6000B fluorescent microscope in the Biomedical Engineering Museum has been repaired, and the brightness can be adjusted from the knob on the body. Welcome to use.
- 2022/11/29 [Instrument relocation] MetaMorph stand-alone version PC1 and PC2 on the fourth floor of the original medical engineering building have been relocated to room 639 of the Library, Information, and Research Building and restored to use.
- 2022/10/17 Education Training Course Handout Archives, if necessary, welcome to download.
- 2022/06/15 [Instrument relocation] Room 639 of the original plan Olympus Fv10i and Olympus IX83 moved to Room 617, reopened since 6/17.
- 2022/05/06 The LSM880 of the Biomedical Engineering Museum has been repaired and returned to use.
- 2022/04/13 Room 640 LSM7MP two-photon laser is ready; online reservations are open now.

Image analysis software- MetaMorph standalone version
Image Analysis Software-MetaMorph Online 10-Person Edition
- the online version can only be used after applying for on-campus IP;
- the location of the stand-alone version Room639, Library, Information & Research Building

Whole Slide Scanning System - Zeiss Axioscan 7
#Certified for self-operation #chargeable use.
Slide Scanner: can scan transmitted light and fluorescent slides in batches, up to 12 standard slides at a time;
- Location: Instrumentation Resource Center, Room 438, Biomedical Engineering Building

Upright Fluorescence Microscope - Leica DM6000B
Certified for self-operation, Motorized Upright Fluorescence Microscope,
- Location: Instrumentation Resource Center, Room 438, 4th Floor, Biomedical Engineering Building

Upright Microscope -Olympus BX63
#Certified for self-operation, Motorized Upright Fluorescence Microscope,
- Location: Room B1, B03, Shou-ren Building

Upright Fluorescence Microscope - Olympus BX61
- Location: Room 639, 6th Floor, Library, Information & Research Building

Motorized Inverted Fluorescence Microscope with Incubation System - Olympus IX83
- Location: Room 617, 6th Floor, Library, Information & Research Building

Super Resolution Microscope with Live-Cell Incubation System - Zeiss Elyra 7
- Location: Room 617, 6th Floor, Library, Information & Research Building

Inverted Laser Scanning Confocal Microscope - Zeiss LSM900 with Airyscan 2
- Location: Room 639, 6th Floor, Library, Information & Research Building

Inverted Laser Scanning Confocal Microscope - Zeiss LSM880 with Airyscan
#Technicians can assist with the operation during office hours. In addition to the Airyscan function, self-operation certification is also available.
Inverted Laser Scanning Confocal Microscope.
- Location: Instrumentation Resource Center, 4th floor, Biomedical Engineering Building

Inverted Laser Scanning Confocal Microscope - Zeiss LSM900 (Upgraded LSM700+)
Inverted Laser Scanning Confocal Microscope;
- Location: Room 640, 6th Floor, Library, Information & Research Building
Two-photon Laser Scanning Microscope - Zeiss LSM7MP
Multi-photon Laser Scanning Confocal Microscope
- Location: Room 640, 6F, Library, Information & Research Building

Inverted Laser Scanning Confocal Microscope - Olympus FV1000
- Location: Room B03, B1 floor, Shou-ren Building

Inverted Laser Scanning Confocal Microscope - Olympus FV10i
Inverted Laser Scanning Confocal Microscope;
- Location: Room 617, 6th Floor, Library, Information & Research Building
| Model | Zeiss Elyra7 |
Zeiss LSM900 |
Zeiss LSM880 |
Olympus FV1000 | Olympus FV10i | ZeissLSM 7MP | ||
|---|---|---|---|---|---|---|---|---|
| Location | Room 617, 6th Floor, Library, Information & Research Building |
Room 639, |
Room 438, 4th Floor, Biomedical Engineering Building |
Room 640, 6th Floor, Library, Information & Research Building |
Room B03, B1 Floor, Shou-ren Building |
Room 617, 6th Floor, Library, Information & Research Building |
Room 640, 6th Floor, Library, Information & Research Building |
|
| Objectives | Air | 10X (Apotome), 20X (Apotome) |
5X, 10X, 20X, | 5X, 10X, 20X, | 5X, 10X, 20X, | 10X, 20X, 40X, | 10X, | -- |
| Oil/water Immersion | 40X oil (Apotome), 63X oil (SIM), 100X oil (TIRF) |
40X(oil), 63X(oil), 100X(oil) |
40X(oil), 40X(water), 63X(oil), 100X(oil) |
40X(oil), 63X(oil), 100X(oil) |
60X(oil), 100X(oil) | 60X(oil) | 20X(water), 40X(water)(VIS-IR), 40X(water)(UV-VIS-IR) |
|
| Laser wavelength (nm) | 405, 488, 561, 642 | 405, 488,561,640 | 405, 440, 458, 488, 514, 543, 594, 633 | 405, 488, 561, 640 | 405, 440, 458, 488, 515, 543, 633, 405(SIM) | 405, 473, 559, 635 | Two-photon laser 690-1040 nm |
|
| Detector | Camera: sCMOS | GaAsp x2, 32-channel GaAsp x1, AiryScan x1 T-PMT x1 |
34-channel QUASAR detectors (PMT x2, 32-channel GaAsp x1), AiryScan x1 T-PMT x1 |
PMT x2, GaAsp x1, ESID x1 |
PMT x3 T-PMT x1 |
PMT x2, T-PMT x1 |
NDD (Non-Descanned Detectors) x2 | |
| Transmission illumination images | BF | BF, DIC | BF, DIC | BF, DIC | BF, DIC | PH | BF, DIC | |
| Application | XY, XYZ, XYZT | V | V | V | V | V | V | -- |
| Tile, multi-area | V | V | V | V | -- | V | -- | |
| Photobleaching | -- | V | V | V | V SIM Laser (405nm) |
-- | V | |
| Lambda scan | -- | V | V | V | V | -- | -- | |
| Live-cell incubation modules (temp. and CO2) |
Full-cover and stage-top incubation system | Full-cover incubation system | Full-cover incubation system | Stage-top incubation system | -- | -- | -- | |
| FL mode (by CCD) | V | V | V | -- | -- | -- | -- | |
| Super Resolution | Lattice SIM | Airyscan 2, Not open for self-operation |
Fast Airyscan, Not open for self-operation |
-- | -- | -- | -- | |
| Terms of use | Certified operation | Specialist operation Or certified to operate by yourself |
Specialist operation Or certified to operate by yourself |
Certified operation | Certified operation | Certified operation | Certified operation, Or contact Dr. Zhu Yexiu (Ext. 66226) |
|
| Model | Olympus BX61 | Leica DM6000 | Olympus BX63 | Olympus IX83 | Zeiss Axioscan7 | |
|---|---|---|---|---|---|---|
| Location | Room 639, 6th Floor, Library, Information & Research Building |
Room 438, 4th Floor, Biomedical Engineering Building |
Room B03, B1 Floor, Shou-ren Building |
Room 617, 6th Floor, Library, Information & Research Building |
Room 438, 4th Floor, Biomedical Engineering Building |
|
| Type | Upright | Upright | Upright | Inverted, with a live-cell incubation system |
Upright, Automatic Slide Scanner |
|
| Sample type | 76x26 mm microscope slide | 76x26 mm microscope slide | 76x26 mm microscope slide | slide, 35mm dish, 86x128mm well plate (Please ask the administrator before using) |
76x26 mm, 76x52 mm microscope slides | |
| Objectives | Air | 10X, 20X, 40X, | 5X, 10X, 20X, | 1.25X, 4X, 10X, 20X, 40X, | 2X, 4X, 10X, 20X, 40X, | 5X/0.25 10X/0.45 20X/0.5, 20X/0.8 40X/0.95 |
| Oil/water Immersion | 100X (oil) | 40X (oil), 63X (oil), 100X (oil) |
100X (oil) | 60X (Silicon oil) | -- | |
| Stage | Manual stage manual XY |
Manual stage manual XY |
Ultrasonic automatic stage, automatic XYZ |
Ultrasonic automatic stage, automatic XYZ |
automatic XYZ, with 3 mounting frames, each can carry 4 76x26mm slides or 2 52x76mm slides. |
|
| Camera | color CCD | monochrome CCD | color CCD | Dual imaging system: color cMOS + monochrome scMOS |
Dual imaging system: color cMOS + monochrome cMOS |
|
| Filter set | DAPI, FITC (510IF), TRITC (575IF) |
DAPI, GFP (525/50), Cy3 (610/75), Red (647/75), Cy5 |
DAPI, GFP (LP510), FITC (BP510-550), TRITC, mCherry, Cy5 |
DAPI, GFP (525/30), FITC-long (LP510), TRITC (607/36), mCherry (645/90), Cy5 (684/24), CFP (472/30), YFP (542/27) |
DAPI (425/30), GFP (514/30), DsRed/mCherry (592/25), Cy5 (681/45), Cy7 (788/38) |
|
| Transmission illumination image | BF (color) | BF (mono), PH (phase contrast) |
BF (color) DIC, PO (dark field) |
BF color, BF mono, PH (4X/10X/20X), DIC (40X/60X) |
BF, PH, DIC | |
| Application | Visible light dyeing (HE stain, etc.) |
V | Grayscale images only | V | V | V |
| Auto multi-channel | -- | V | V | V | V | |
| Automatic multi-position | -- | -- | V | V | V | |
| Tile image | Requires additional software assistance | Requires additional software assistance | V automatic with a focus map |
V automatic with a focus map |
V automatic detection, focusing, and stitching |
|
| Automatic Z-stack | V | V | V | V | V | |
| Terms of use | Certified operation, Free for faculties and students on campus |
Certified operation, Free for faculties and students on campus |
Certified operation, Free for faculties and students on campus |
Certified operation, On-campus usage fee: NTD 50 per half-hour |
Certified operation, On-campus usage fee: NTD 150 per half-hour |
|
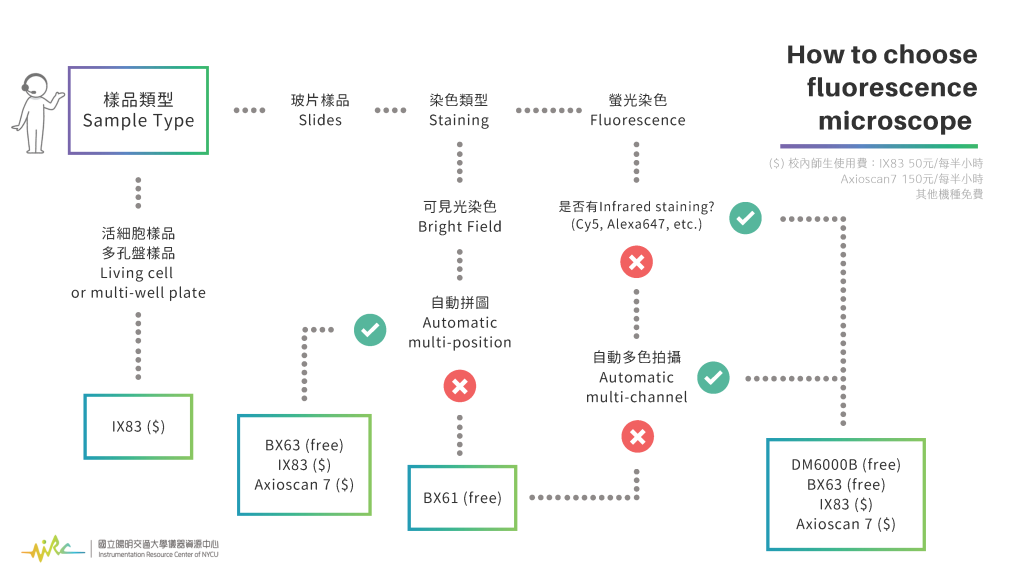
- Selection Basis
- * Sample Type: Slides
- > Fluorescence Staining
- -- with infrared staining and automatic multi-color acquisition: DM6000B (free), BX63 (free), IX83 ($), Axuiscan7 ($)
- -- No need for infrared staining and automatic multi-color acquisition: BX61 (free)
- > Immunohistochemical staining
- -- With automatic multi-area acquisition and stitching: BX63 (free), IX83 ($), Axioscan7 ($)
- -- No need for automatic multi-area acquisition: BX61 (free)
- > Fluorescence Staining
- * Sample Type: Living cell or multi-well plate: IX83 ($)
- ($) Usage Fee for NYCU faculties and students:
- > IX83: NTD 50 per half-hour
- > Axioscan7: NTD 150 per half-hour
- > Other models are free
- In addition to the imaging core facility education and training courses organized by the center, it is recommended that you bring your samples for scheduled teaching and certification exams (experimental data can be taken away). The charges will be the same as for general use.
- If you have the following qualifications, you will be charged the same fee as NYCU faculty and students:
- Branch school
- Joint-appointed faculty
- Affiliated hospitals
- Projects are done in cooperation with and under the control of the university.
- If you have the following qualifications, the fee will be the same as that of a cooperative educational unit:
- Adjunct Faculty
- Teaching hospitals (Veterans General Hospital, Taipei City Hospital, Far Eastern Memorial Hospital, Cheng Hsin General Hospital, etc.)
- University System of Taiwan (UST)
- Companies participate in the academia-industry cooperation center of NYCU
| Fluorescence Microscope |
Slide Scanner | Upright type | Inverted type (For living cells) |
Based on Confocal Microscope |
|---|---|---|---|---|
| Model | (Z) Axioscan 7 | (L) DM6000B (O) BX61 (O) BX63 |
(O) IX83 | (Z) LSM880 with Airyscan (Z) LSM900 with Airyscan2 |
| Fees for NYCU faculty and students | 300 | 0 | 100 | 300 |
| Fees for cooperative educational units | 450 | 50 | 150 | 450 |
| Fees for other unit | 600 | 100 | 200 | 600 |
| Remarks |
|
Users must complete confocal microscope certification to use. | ||
| Confocal Microscopes |
(Z) LSM880 Office hours |
(Z) LSM880 Night |
(Z) LSM900* Office hours |
(Z) LSM900* Night |
(Z) LSM900 (LSM700+) |
(O) FV1000 | (O) FV10i | (Z) LSM7MP |
|---|---|---|---|---|---|---|---|---|
| Restricted to Certified Users | -- | V | -- | V | V | V | V | V |
| Fees for NYCU faculty and students | 900 | 500 | 800 | 600 | 600 | 500 | 300 | 1,000 |
| Fees for cooperative educational units | 1,350 | -- | 1,200 | -- | 900 | 750 | 450 | 1,500 |
| Fees for other unit | 1,800 | -- | 1,600 | -- | 1,200 | 1,000 | 600 | 2,000 |
| Remarks |
|
|||||||
| Special optical microscope | Super-Resolution Microscope with Lattice SIM² and Incubation System |
|---|---|
| Model | (Z) Elyra 7 |
| Fees for NYCU faculty and students | 1,300 |
| Fees for cooperative educational units | 1,950 |
| Fees for other unit | 2,600 |
| Remarks |
|
- Ms. Pei-Jun Chen
Ext: 65980 / Instrument Resource Center, 4th Floor, Biomedical Engineering Building
pjchen3@nycu.edu.tw
- Ms. Yung-yu Lu
Ext: 66185 / Room 639, 6th Floor, Library, Information & Research Building
yungyu@nycu.edu.tw












 中文
中文


